Abstract
Background: Therapeutic plasma exchange (TPE), is used in various hematological disorders. Per the American Society for Apheresis (ASFA) guidelines, TPE is a Category III indication in the management of ITP. Category III means the optimum role of apheresis therapy is not established and decision making should be individualized. This study evaluates nationwide TPE use in hospitalized adults with a primary admission diagnosis of ITP.
Study Design and Methods: Hospitalizations with ITP as the primary admitting diagnosis were analyzed from the 2010-2014 National Inpatient Sample (NIS). NIS is the largest all-payer inpatient database for United States hospitalizations. Due to limited TPE usage in pediatric ITP hospitalizations (below NIS threshold analysis minimum ten hospitalizations), children were excluded from these analyses. Any hospitalizations with thrombotic thrombocytopenic purpura (TTP) as a secondary diagnosis were also excluded. Univariate and multivariable logistic regressions were used to determine predicting factors of TPE and clinical outcomes in ITP patients undergoing TPE. Sampling weights were applied to generate nationally representative estimates.
Results: From 2010-2014, analyzing hospitalizations with ITP listed as 'one of all diagnoses' during hospital course, there were total of 282,285 admissions of which 1,452 admissions (0.6%) reported TPE. 60,940 adult hospitalizations had ITP as the primary diagnosis. Of these 60,940 primary ITP admissions, 1.04% admissions (n=635) reported TPE during the hospital course. In the 635 ITP admissions undergoing TPE, 76% of TPE procedures were initiated within the first 48 hours. Most subjects getting TPE were the highest disease severity class: Major (30.4%) and Extreme severity (49.5%, all-patients refined diagnoses-related-groups (APRDRG) severity of illness subclass). Among ITP admissions with TPE, the following occurred: platelet transfusions (20.2%), hemodialysis (9.9%), mechanical ventilation (9.4%), IVIG (8.7%), and splenectomy (3.0%). There were approximately 50% co-morbidities among ITP admissions undergoing TPE: acute kidney failure (27.3%), hemolytic anemia (11.1%), acute respiratory failure (10.6%), systematic lupus erythematosus (4.8%), human immunodeficiency virus (2.4%), and hepatitis C (2.4%). After excluding acute kidney injury and acute renal failure as co-morbidities, the % of ITP admissions undergoing TPE decreased to 0.8%.
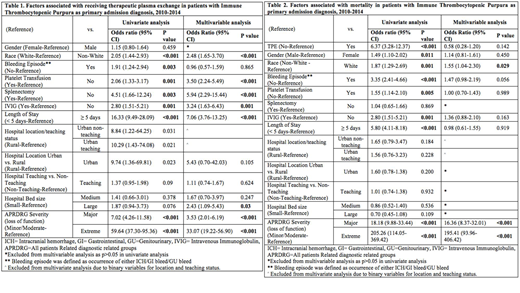
Among all ITP admissions, 12.3% reported at least one bleeding complication (gastrointestinal, 6.2%, genitourinary, 5.3%, and intracerebral hemorrhage, 1.05%). Among ITP hospitalizations with TPE, 20.9% cases reported at least one bleeding complication (gastrointestinal bleeding, 9.4%, genitourinary bleeding, 11.86%, and intracerebral hemorrhage. 3.07%) (p<0.05). After multivariable analysis, underlying severity of illness remained the most significant predictor of undergoing TPE (p<0.001) [Table 1]. Admissions categorized as major (adjOR=3.53, 95%CI=2.01-6.19, p<0.001) and extreme severity of illness (adjOR=33.07, 95%CI=19.22-56.90, p<0.001) had substantially higher odds of undergoing TPE than less severe hospitalizations. Admissions with TPE also had significantly, substantially longer mean length of stay in the hospital (p<0.001). All-cause mortality was 1.4% among all ITP hospitalizations and 7.8% in ITP hospitalizations with TPE. However, patients with TPE showed neither an improvement nor a worsening in the adjusted odds of all-cause mortality (p-value=0.142) [Table 2] or bleeding status (p-value=0.755).
Conclusion: TPE was reported in about 1% of hospitalizations with ITP as the primary diagnosis in this nationally representative sample between 2010-2014. TPE was performed in patients with highest severity of underlying illness, more significant bleeding, and a high (50%) rate of comorbidities. No clear associations with improvement or worsening of mortality or bleeding outcomes was seen in ITP hospitalizations reporting but neither was there any evidence of increased bleeding or morbidity with the procedure.
Bussel:Prophylix: Consultancy, Research Funding; Momenta: Consultancy; Amgen Inc.: Consultancy, Research Funding; Uptodate: Honoraria; Novartis: Consultancy, Research Funding; Protalex: Consultancy; Rigel: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal